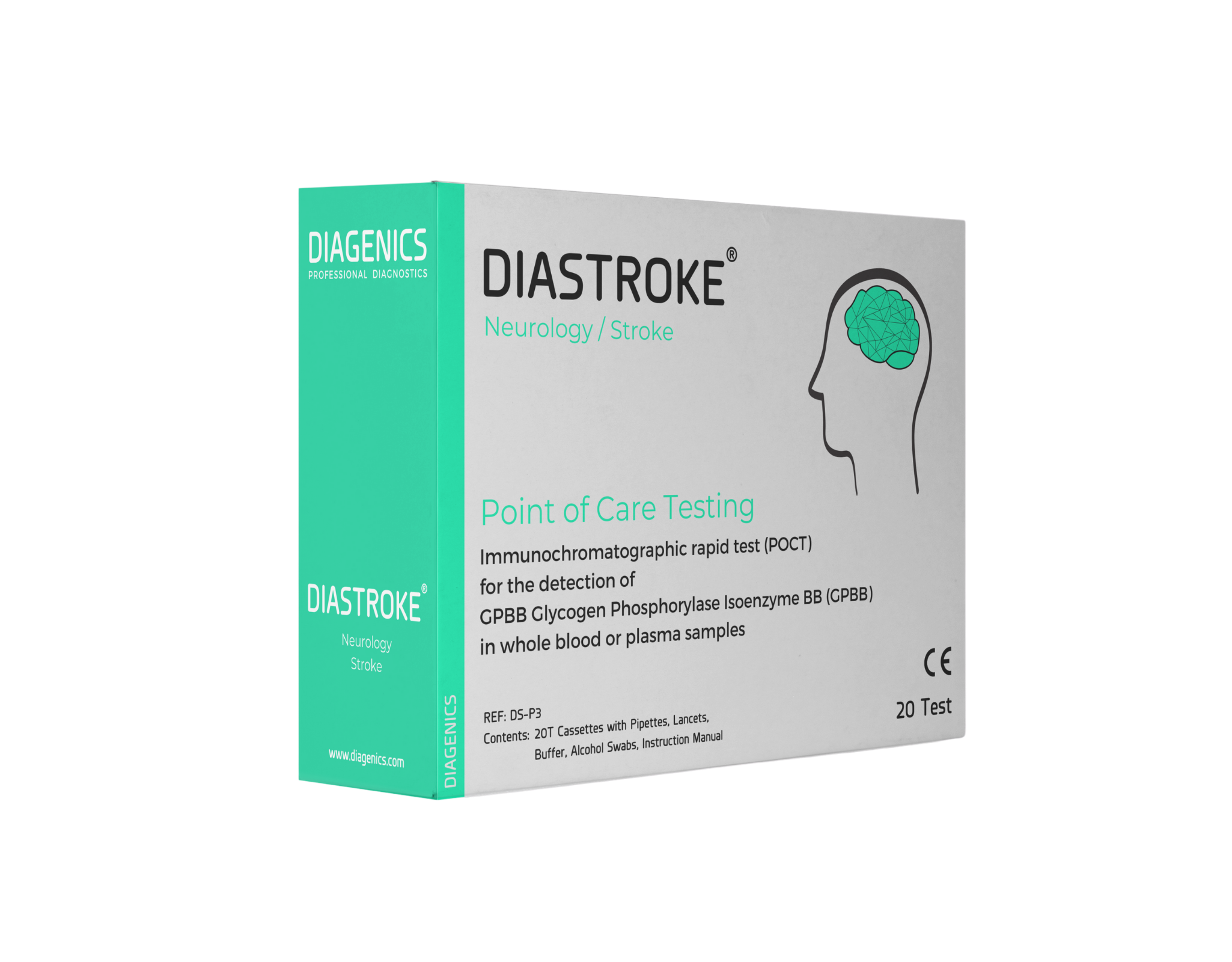
POCT
Rapid test (point-of-care test) early detection of Stroke
Know your symptoms
The point-of-care test (POCT) is used in emergency medicine as a quick and qualitative test for a therapy decision-making basis. Point-of-care tests are advantageous due to their quick results, as a diagnosis can be made immediately without the help of a laboratory or an existing diagnosis is supported by the test result. The DIASTROKE® POCT is a strip test that works on the basis of GPBB as a biomarker for strokes.
The monoclonal catch antibody for GPBB is applied in narrow tracks on the multilayered strip. Another antibody (detect antibody) is applied to the strip with a special buffer solution after the drop of blood. This antibody is coupled with an enzyme that causes the color change. If the blood sample runs along the absorbent strip onto the antibody track, GPBB is bound by catch antibodies and made visible with the help of the enzyme reaction. As little as 50 µl is enough to take a solid measurement. A result is available after just 15 minutes. To date, there has been no early and reliable biomarker test for stroke diagnosis. Therefore, it is currently very difficult for doctors to diagnose or exclude a stroke without time-consuming and costly diagnostics.
DIAGENICS offers its patented blood test based on GPBB for a group of indications that currently has no alternative. DIASTROKE® is therefore trend-setting in the diagnosis of strokes. The goal of DIAGENICS is to establish DIASTROKE® as the standard for the diagnosis and monitoring of cerebral ischemia. Large-scale studies are currently being planned to verify the sensitivity and specificity of the DIASTROKE® POCT in comparison to the ELISA. The test is easy, quick, safe and reliable. Marketing of the product will be initiated worldwide.